Which Best Describes the Acid Base Classification of Ammonia
Ammonia D vitamin C. D When the acidity of a solution increases the pH increases.

2 Polar Covalent Bonds Acids And Bases Ppt Download
Eddie must decide where add baking soda pH 9.

. Which of the following compositions of cleaners is the best choice if you have hard water. C When an acid reacts with a carbonate carbon dioxide is given off. Strong acids and bases such as hydrochloric acid and potassium hydroxide have a high propensity to dissociate in water and become ionised in solution.
Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety. An acid is a substance which ionises or dissociates in water to produce hydrogen ions H. A When a base reacts with an ammonium salt ammonia is given off.
Ammonia is a BasicSo option B is your answerHope this helps. Reaction is given below Reaction is given below Reaction of Boron trifluoride Lewis acid with fluoride ion Lewis base Octet of Boron in Boron trifluoride BF 3 is incomplete so it acts as a very good Lewis acid and combines with fluoride ion and. Large macromolecules or polyanions like proteins.
What class of compounds is primarily responsible for the pleasant smell of many fruits. Muriatic acid is industrial hydrochloric acid HCl. View the full answer.
View acides bases and salts QS 1pdf from PS 147 at Institute of Education Main Campus Khairpur. The ammonium ion is its conjugate acid - it can release that hydrogen ion again to reform the ammonia. Since it has come from the base ammonia it is called as a conjugate acid of base ammonia.
NH3 aq 3H20 I N aq 3H30 aq NH3 aq H2O l - NH4 aq OH aq NH3 aq N3- aq 3H aq NH3 aq H2O l NH4 aq OH aq NH3 aq 3H2O 1 N3- aq 3H30 aq. NH3 normally found as a gas it is caustic and harmful in longterm exposure. The hydroxide ion can accept a hydrogen ion to reform the water.
NH3 is a weak base with pH 11 at standard conditions but it is also considered amphoteric which means it can act as both acid and base under different conditions. They are referred to as weak acids or weak bases. Its structure is tetrahedral.
This even happens in. Ammonia is a foundation since the water accepts hydrogen ions. For this reason ammonia is considered basic because its nitrogen atom has an electron pair that readily accepts a proton.
The strength of an acid or base depends on _____. NH 4 like HCl can donate a proton and hence an acid. Is muriatic acid same as ammonia.
The Swedish chemist Arrhenius proposed the following definition of an acid. NH_3aq H_2Ol NH_4aq OH-aq NH_3aq 3H_2Ol N3-aq 3H_2Oaq NH_3aq 3H_2Ol N aq 3H_2O aq NH_3aq rightarrow N aq 3H aq What is the net ionic equations for the complete neutralization of. An acid a base a neutral 2 See answers Advertisement Advertisement SJ2006 SJ2006 Ammonia is a Basic So option B is your answer.
Like water liquid ammonia undergoes molecular autoionisation to form its acid and base conjugates. A how many hydrogen atoms are contained in each molecule of the acid or base. Ammonia often functions as a weak base so it has some buffering ability.
Which best describes the chemical behavior of amines. Water behaves as an acid and the hydroxide ion is the conjugate base. Some of the ammonia will react and from NH4OH or ammonium hydroxide a base.
Similarly Cl like ammonia can accept a proton and hence a base. Hence multiple often redundant pathways and processes exist to control systemic pH. Which of the following reactions best describes the acid-base properties of ammonia NH3 in aqueous solution.
A long hydrocarbon chain with sodium salt B long hydrocarbon chain with potassium salt. A crude nonacidic product mixture dissolved in chloroform contains acetic acid. For example hydrochloric acid HClaq is a solution of hydrogen chloride in water obtained by dissolving pure hydrogen.
2 NH 3 NH 4 NH 2. _ acids bases and salts CHEMISTRY OXFORD IGCSE HEBA. 12022016 Chemistry High School answered Ammonia is _____.
Eddies chemistry teacher has asked the class to add various household substances to this scale of acids and bases. It is a gas NH3. NH3 under suitable condition act as a weak base and accepts H and forms its conjugate acid NH4 and under different condition NH3 will act as an extremely weak acid and give away H ion to form.
What is the definition of an acid and a base. Muriatic acid and ammonia. However ammonia is classified as a weak base which is a chemical compound that doesnt completely break apart into ions in an aqueous solution.
The water is acting as an acid and its conjugate base is the hydroxide ion. Which of the following reactions best describes the acid-base properties of ammonia NH_4 in aqueous solution. B When an acid reacts with a base neutralisation takes place.
The reaction of H Lewis acid and NH 3 Lewis base Ammonia combines with hydrogen ions and forms ammonium ions. A base is any molecule that accepts a proton while an acid is any molecule that releases a proton. Acid-base homeostasis is critical for normal physiology and health.
Ammonia is used in nitric acid production as a fertilizer and a cleaning solution. The ammonium ion is the conjugate acid this hydrogen ion should be produced again in order to reform the ammonia. However if you bubble ammonia into water you get a reversible reaction.
Both the reaction of an acid chloride with an amine and the reaction of an acid anhydride with ammonia are correct. Amino Acids Definition Structure Properties Classification. Theories of acid-base indicators as Weak acids and weak bases such as acetic acid and ammonia do not dissociate completely in water.
A biomolecule sometimes known as a biological molecule is a term that refers to molecules found in living things that are required for one or more biological processes such as cell division morphogenesis or development. Derangements in acid-base homeostasis however are common in clinical medicine and can often be related to the systems involved in acid-base transport in the kidneys. It is considered as the conjugate base of the acid HCl.
Ammonia in and of itself is neither a base nor an acid. Due to the NH3 molecules polarity and its ability to form hydrogen bonds ammonia is strongly soluble in water. Ammonia is made out of one nitrogen and three hydrogen atoms.
Looking at it from the other side the ammonium ion is an acid and ammonia is its conjugate base. Eddie should insert baking soda pH. Shifts in pH will cause more or fewer ammonium cations NH 4 and amide anions NH 2 to be present in solution.
Ammonia is a base NH3. The Bronsted Lowry transfer can be written as-.
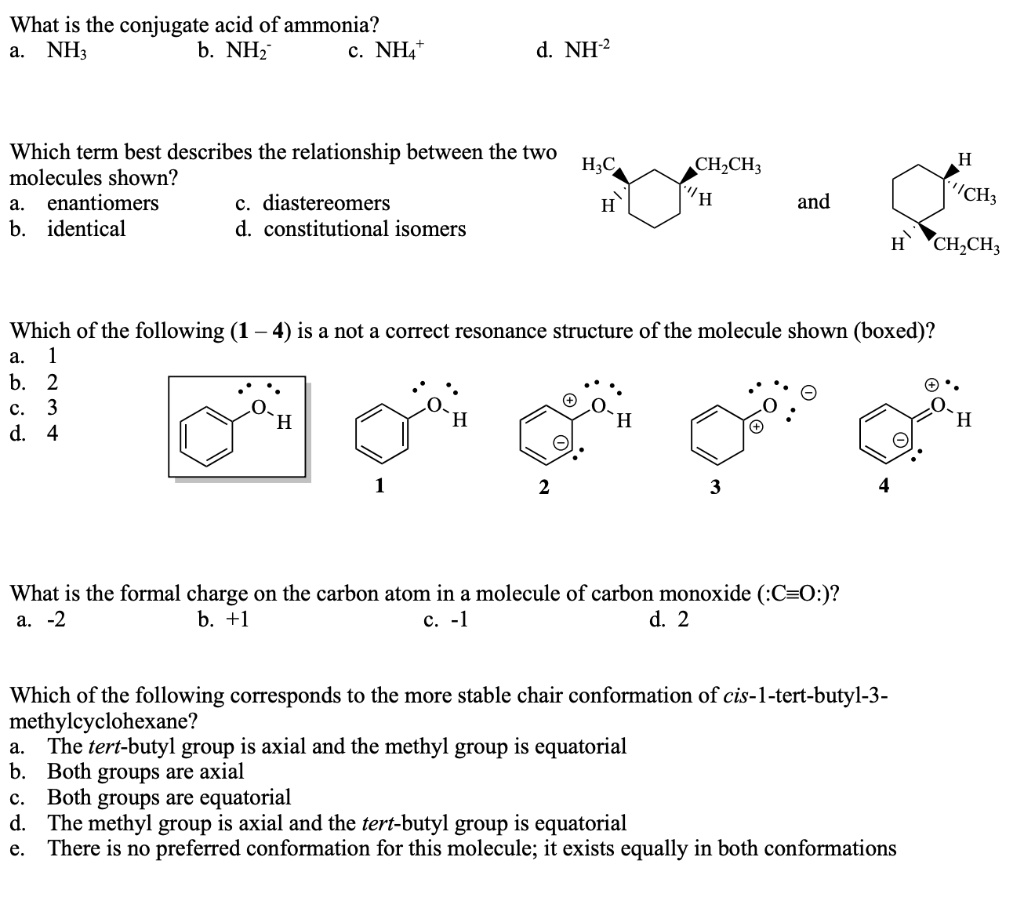
Solved What Is The Conjugate Acid Of Ammonia Nh Nhz Nh4 Which Term Best Describes The Relationship Between The Two H C Molecules Shown Enantiomers Diastereomers B Identical Constitutional Isomers Ch Ch H And

Solved Which Of The Following Reactions Best Describes The Chegg Com
Solved Tools None U O Na 0 H2 O Nh3 O Make Ammonia Chegg Com
No comments for "Which Best Describes the Acid Base Classification of Ammonia"
Post a Comment